
SUCCESS PTA: 12-Month Data from SELUTION SLR™ DEB
www.radcliffevascular.com
Dec. 15, 2025, 8:37 a.m.
12 Month Outcomes of the SUCCESS PTA Study show consistent safety and effectiveness in claudicant and CLTI patients when using the SELUTION SLR™ drug eluting balloon.
Share on

JCI - A smooth muscle cell lncRNA controls angiogenesis in chronic limb-threatening ischemia through miR-143-3p/HHIP signaling
www.jci.org
Dec. 7, 2025, 7:33 p.m.
RNA-seq identified downregulation of the Hedgehog signaling pathway in CARMN-KO models and revealed that CARMN regulates this pathway through its downstream miRNA, miR-143-3p, which targets Hedgehog-interacting protein (HHIP), an antagonist of Hedgehog signaling. Delivery of HHIP-specific siRNA or miR-143-3p mimics rescued EC angiogenic defects and improved blood flow recovery in both CARMN-KO and WT mice. These findings underscore the critical role of CARMN in modulating angiogenesis through the miR-143-3p-HHIP-Hedgehog signaling axis, providing insights into SMC-EC interactions and potential therapeutic strategies for CLTI.
Share on

Abbott's Esprit BTK receives Health Canada approval for treating below-the-knee CLTI
vascularnews.com
Nov. 30, 2025, 10:07 p.m.
Until this recent Health Canada approval for the Esprit BTK system, Abbott notes that the standard of care has been balloon angioplasty. However, the company notes that treatment with balloon angioplasty alone can have poor short- and long-term results, and—in many instances—the vessels do not remain patent, requiring additional treatment.
Share on

Three-year data support drug-eluting resorbable scaffold as “durable, effective endovascular treatment option” for CLTI
vascularnews.com
Nov. 30, 2025, 10:05 p.m.
Sahil Parikh (Columbia University Irving Medical Center, New York, USA) reported that, at three years, Esprit BTK demonstrated a 33% relative improvement in the composite primary efficacy endpoint of primary patency and limb salvage compared to percutaneous transluminal angioplasty (PTA; 59.5% vs. 44.8%; p=0.0025 log rank test). Parikh added that the Esprit BTK was also associated with significantly lower rates of binary restenosis (38% vs. 49%; p=0.018 log rank test), and lower clinically driven target lesion revascularisation (CD-TLR) rates, though the latter did not reach statistical significance (10.2% vs. 18.4%; p=0.052 log rank test). Furthermore, the presenter shared that safety outcomes, the composite of freedom from major adverse limb events at three years, and perioperative death at 30 days were similar between the two arms (90.8% vs. 94.2%; p=0.23 log rank test).
Share on

No patient benefit from drug-coated balloons in major Swedish trials
www.gu.se
Nov. 30, 2025, 3:30 p.m.
Drug-coated balloons and stents don’t reduce the risk of amputation or improve quality of life for people with peripheral artery disease in the legs. That’s the takeaway from two major clinical trials led by researchers at the University of Gothenburg.
Share on
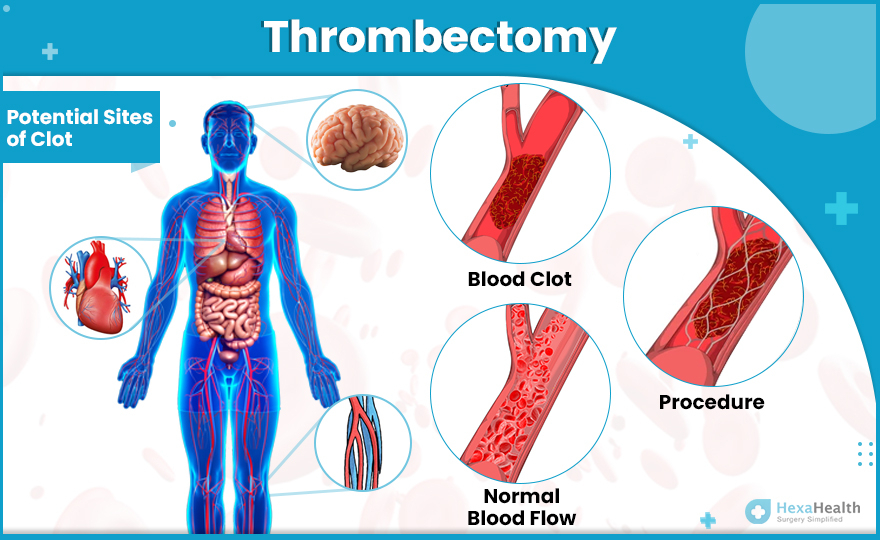
Procedural duration “exerts a greater influence” on post-thrombectomy functional outcomes compared to prehospital delays
neuronewsinternational.com
Oct. 20, 2025, 11:07 a.m.
“Endovascular thrombectomy has transformed acute ischaemic stroke care, with onset-to-puncture [OTP] time widely recognised as a critical determinant of outcome,” the authors write, outlining the backdrop to their study. “However, emerging evidence suggests that in-hospital procedure time—from arterial puncture to final recanalisation—may have an equally or more significant impact.”
Share on
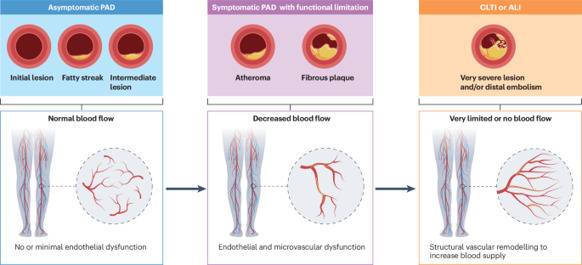
Peripheral artery disease
www.nature.com
Sept. 22, 2025, 10:21 a.m.
For patients with compromised limb viability, such as acute and chronic limb-threatening ischaemia, or severe functional impairment that does not improve with exercise training, lower extremity revascularization is recommended. Given the complexity of PAD management, a multidisciplinary vascular team is required to achieve the best individualized treatment. Further research efforts should focus on reducing ischaemic events and health disparities and on optimizing the implementation of GDMT and exercise therapy, as well as improving the quality of life in patients with PAD.
Share on
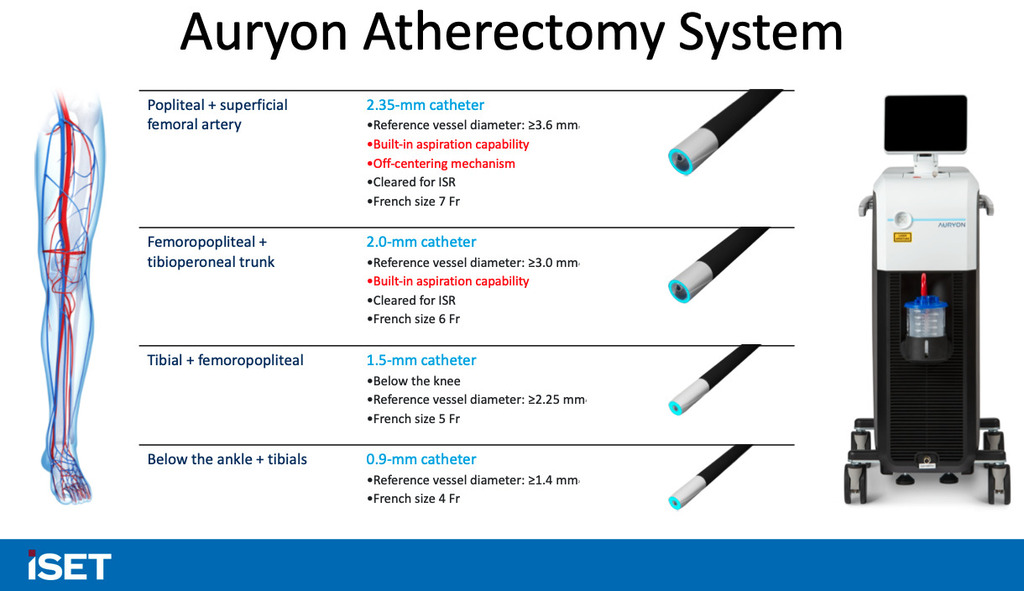
First patient enrolled in AngioDynamics' AMBITION BTK trial for CLTI
vascularnews.com
Aug. 8, 2025, 1:36 p.m.
A press release details that AMBITION BTK is a prospective, multicentre, randomised controlled trial (RCT) designed to investigate the clinical safety and effectiveness of the Auryon atherectomy system combined with standard balloon angioplasty, compared to balloon angioplasty alone, in treating infrapopliteal lesions in patients with CLTI. The primary endpoint will be evaluated using a win-ratio approach, comparing the two treatment groups based on the following components in a hierarchical manner at 12 months: freedom from major amputation, freedom from clinically driven target lesion revascularisation (CD-TLR), and primary patency.
Share on

US FDA grants de novo clearance for Reflow Medical's Spur peripheral retrievable stent system
vascularnews.com
Aug. 8, 2025, 1:33 p.m.
According to Reflow Medical, the Spur stent system is the first and only retrievable stent system that features a self-expanding stent with an integrated dilatation balloon catheter on an over-the-wire system. It is designed for controlled lesion penetration and treatment through a series of radially expandable spikes. Known as retrievable scaffold therapy (RST), the spikes on the Spur stent penetrate the lesion to increase the acute luminal diameter and modify the lesion morphology to change vessel compliance and reduce vessel recoil effect.
Share on
New guidance aims to improve blood clot prevention in chronic limb-threatening ischemia patients
www.news-medical.net
Aug. 8, 2025, 1:32 p.m.
A new statement from leading heart and blood vessel experts in Europe is providing clinical guidance for treatments to prevent blood clots in patients with a serious condition called chronic limb-threatening ischemia (CLTI), after they have had procedures to restore blood flow in their lower limbs. The statement, and the systematic review it is based on, comes from the European Society of Cardiology (ESC) Working Group on Aorta and Peripheral Vascular Diseases and Working Group on Cardiovascular Pharmacotherapy.
Share on

StentIt launches first-in-human trial of bioresorbable stent to treat below-the-knee CLTI
vascularnews.com
Aug. 8, 2025, 1:32 p.m.
StentIt’s RFS device is a bioresorbable stent built from microfibers, providing structural support to instantly open, and facilitate the reconstruction of the artery. Due to the porous design of the implant, patient’s own cells infiltrate into the mesh, triggering the formation of new vascular tissue. While the artery is being reconstructed from the inside-out, the synthetic implant gradually resorbs and ultimately disappears over time.
Share on

CX 2025: Bioresorbable scaffold technology has ‘potential to be a new paradigm for femoropopliteal endovascular intervention’
cardiovascularnews.com
May 5, 2025, 6:10 a.m.
In Wednesday’s peripheral arterial programme, which hosted several podium-first presentations, lower limb drug-eluting technologies took centre stage—signalling a potential shift in the treatment paradigm for chronic limb-threatening ischaemia (CLTI). Investigators shared compelling new data from studies ranging from first-in-human trials to large-scale registries, which vie to redefine durability and safety, as well as cost-effectiveness, in the peripheral arterial treatment landscape.
Share on

How is Critical Limb Ischemia treated?
aadicura.com
March 17, 2025, 8:22 a.m.
Critical Limb Ischemia (CLI) is a condition manifested by pain at rest, a non-healing wound, or gangrene. It occurs due to severe blockages in the arteries of a person’s lower extremities/limbs (legs), which markedly reduces blood flow. This condition is a serious form of Peripheral Arterial Disease or PAD that is caused by the buildup of fats, cholesterol or some other substances in and around your artery walls.
Share on
Two-year LIFE-BTK data show sustained benefits of drug-eluting resorbable scaffold for below-the-knee arteries
cardiovascularnews.com
Dec. 7, 2024, 6:30 a.m.
Presented today, late-breaking data from the second year of the LIFE-BTK clinical trial demonstrate the long-term effectiveness of the US Food and Drug Administration (FDA)-approved Esprit BTK everolimus-eluting resorbable scaffold system (Abbott Vascular) in patients with the most severe form of below-the-knee (BTK) peripheral arterial disease (PAD). The data show that the Esprit BTK offers sustained benefits over balloon angioplasty with fewer repeat procedures at two years.
Share on
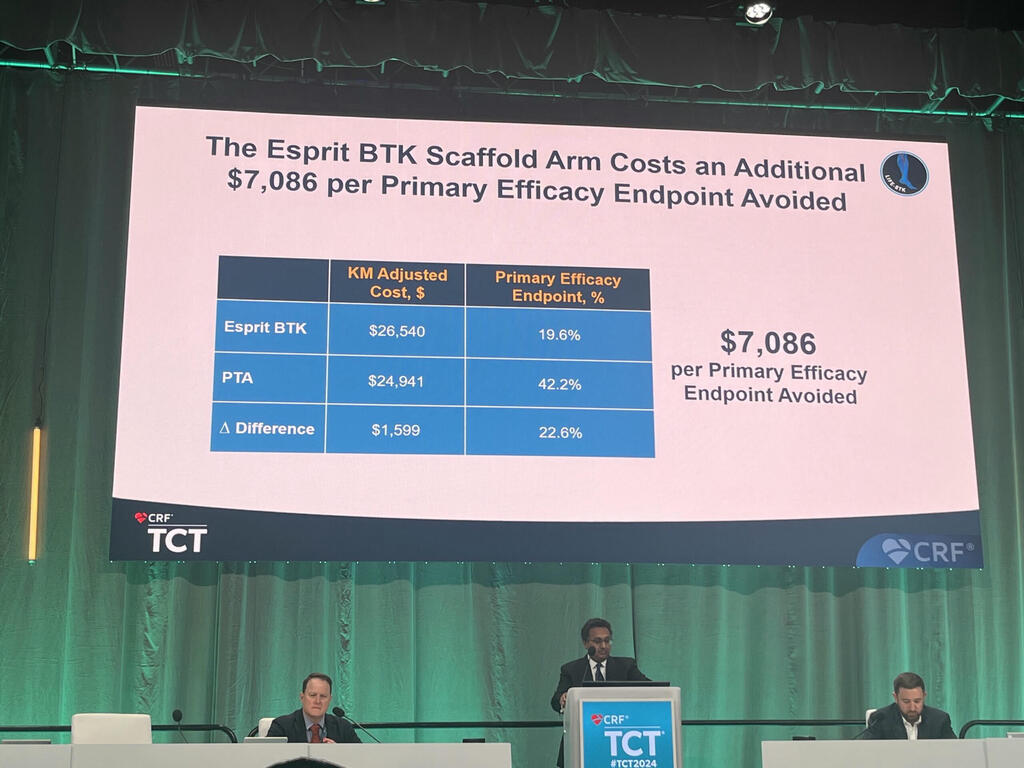
TCT 2024: Drug-eluting resorbable scaffold proves cost effective at one year in LIFE-BTK analysis
cardiovascularnews.com
Dec. 7, 2024, 6:27 a.m.
Using the most conservative delta between the two groups in terms of the primary efficacy endpoint at 365 days, not inclusive of the treatment window, the Esprit BTK scaffold costs an additional US$7,086 per primary efficacy endpoint avoided. This led Parikh to report that the Esprit BTK scaffold achieves a 64% probability of cost-effectiveness at a US$10,000 willingness-to-pay threshold compared to angioplasty. As a result of this, he averred: “The use of Esprit BTK at one year alone is likely to be cost effective.”
Share on

Camouflaging endovascular stents with an endothelial coat using CD31 domain 1 and 2 mimetic peptides
jvsvs.org
Sept. 2, 2024, 3:07 p.m.
Using peptides replicating the membrane-distal portion of CD31, which is prominently exposed on the inner side of healthy vessels, can simulate the surface of a healthy endothelium. This approach effectively prevents the deposition and activation of blood platelets and leukocytes, which are abnormally prolonged by the presence of a foreign body at sites of endovascular stent implantation, and promotes the acquisition of physiological phenotype by the ECs covering the surface.
Share on
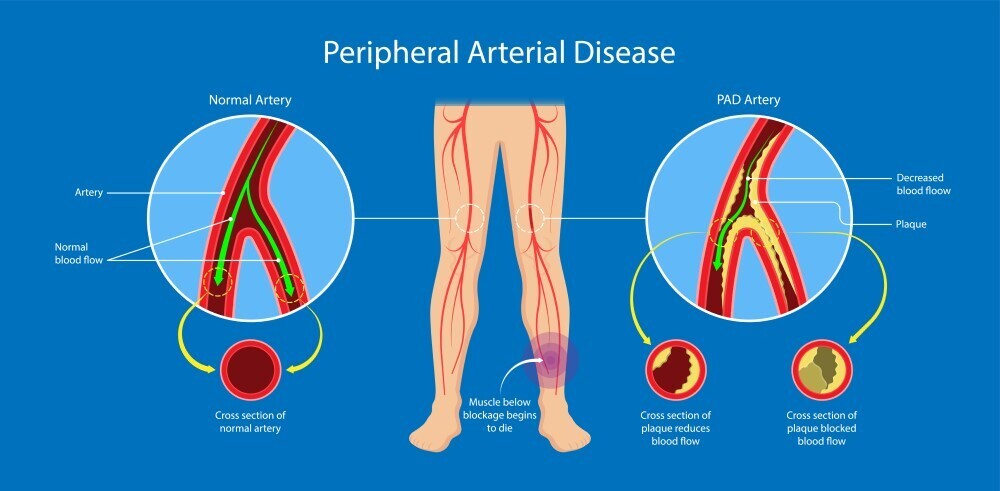
New Horizons in Peripheral Artery Disease
academic.oup.com
Aug. 15, 2024, 10:45 a.m.
Peripheral artery disease (PAD) is the lower limb manifestation of systemic atherosclerotic disease. PAD may initially present with symptoms of intermittent claudication, whilst chronic limb-threatening ischaemia (CLTI), the end stage of PAD, presents with rest pain and/or tissue loss. PAD is an age-related condition present in over 10% of those aged ≥65 in high-income countries. Guidelines regarding definition, diagnosis and staging of PAD and CLTI have been updated to reflect the changing patterns and presentations of disease given the increasing prevalence of diabetes.
Share on
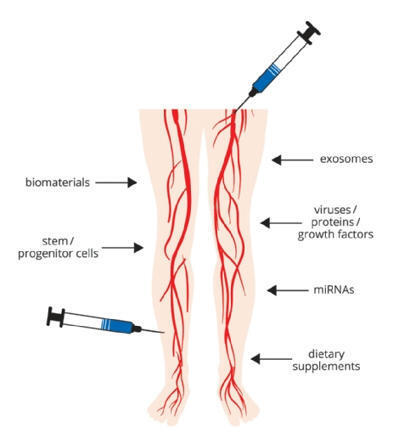
New horizons in the treatment of lower extremity arterial disease
www.escardio.org
Aug. 15, 2024, 10:44 a.m.
Despite medical and technical progress, therapy of lower extremity arterial disease (LEAD) remains limited by subsequent disease progression and ultimate limb ischaemia. Even today, interventional and surgical revascularisation does not enable long-term success in all patients, and/or may not be technically feasible in end-stage LEAD anymore. Subsequent intractable claudication, impaired quality-of-life and work capacity, limb loss, and associated morbidity and mortality have thus generated impetus for alternative therapies.
Share on
Humacyte's ATEV granted US FDA Regenerative Medicine Advanced Therapy designation for advanced PAD
vascularnews.com
Aug. 15, 2024, 10:41 a.m.
Humacyte recently announced it has been granted Regenerative Medicine Advanced Therapy (RMAT) designation from the US Food and Drug Administration (FDA) for its investigational acellular tissue engineered vessel (ATEV), designed to treat patients with advanced peripheral arterial disease (PAD). This RMAT designation was granted at the same time as the FDA cleared a new Investigational New Drug (IND) application for the PAD indication for ATEV, formerly referred to as the human acellular vessel (HAV).
Share on
A subpopulation of tissue remodeling monocytes stimulates revascularization of the ischemic limb
www.science.org
Aug. 14, 2024, 8:07 a.m.
Despite decades of effort aimed at developing clinically effective cell therapies, including mixed population mononuclear cells, to revascularize the ischemic limb, there remains a paucity of patient-based studies that inform the function and fate of candidate cell types. In this study, we showed that circulating proangiogenic/arteriogenic monocytes (PAMs) expressing the FcγIIIA receptor CD16 were elevated in patients with chronic limb-threatening ischemia (CLTI), and these amounts decreased after revascularization. Unlike CD16-negative monocytes, PAMs showed large vessel remodeling properties in vitro when cultured with endothelial cells and smooth muscle cells and promoted salvage of the ischemic limb in vivo in a mouse model of hindlimb ischemia. PAMs showed a propensity to migrate toward and bind to ischemic muscle and to secrete angiogenic/arteriogenic factors, vascular endothelial growth factor A (VEGF-A) and heparin-binding epidermal growth factor.
Share on