
Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility
www.frontiersin.org
April 24, 2024, 2:32 a.m.
The interaction of biomaterials with blood leads to cellular as well as humoral reactions, which can result in an unwanted inflammation and activation of coagulation and/or fibrinolysis. Thus, the development of biomaterials with an improved hemocompatibility increases the tolerability and minimizes unwanted side effects, such as thrombus formation. Therefore, during the development of new blood-contacting medical devices, not only the mechanical and chemical characteristics should play an important role, but also the hemocompatibility. Furthermore, to prove the safety and reliability of new products, hemocompatibility analyses should include appropriate references and follow the ISO 10993-4 standard.
Share on

An In Vitro Blood Flow Loop System for Thrombogenicity Evaluation of Medical Devices and Biomaterials
www.fda.gov
April 24, 2024, 2:31 a.m.
There is currently no standardized test method for in vitro dynamic thrombogenicity assessment of medical devices and biomaterials. This tool will effectively enable users to differentiate device materials with different thrombogenic potentials against standard negative and positive control materials and a marketed comparator device with known thrombogenicity profile.
Share on

Comparison of animal and human blood for in vitro dynamic thrombogenicity testing of biomaterials
onlinelibrary.wiley.com
April 24, 2024, 2:29 a.m.
These results demonstrated that multiple animal blood sources (particularly donor ovine and bovine blood) may be suitable alternatives to fresh human blood for dynamic thrombogenicity testing when appropriate control materials and donor-specific anticoagulation levels are used.
Share on
Impact of Frailty in Percutaneous Mitral & Tricuspid Valve Repair
www.physiciansweekly.com
April 24, 2024, 2:22 a.m.
While frailty in mitral/tricuspid valve repair patients was linked to higher bleeding, infection, and hospital stay, it independently predicted long-term mortality, suggesting mechanisms beyond complications need further investigation to help this vulnerable population.
Share on

What are the benefits of valve replacement surgery?
medserg.com
April 24, 2024, 2:21 a.m.
The progress made in heart valve replacements is an incredible advancement for the medical world. By further expanding their research and technology, doctors will be able to help a larger population of patients with fewer risks or complications associated with the procedure.
Share on

Mitral Annular Disjunction: Review of an Increasingly Recognized Mitral Valve Entity
pubs.rsna.org
April 24, 2024, 2:19 a.m.
Mitral annular disjunction (MAD) refers to atrial displacement of the hinge point of the mitral valve annulus from the ventricular myocardium. MAD leads to paradoxical expansion of the annulus in systole and may often be associated with mitral valve prolapse (MVP), leaflet degeneration, myocardial and papillary muscle fibrosis, and, potentially, malignant cardiac arrhythmias. Patients with MAD and MVP may present similarly, and MAD is potentially the missing link in explaining why some patients with MVP experience adverse outcomes.
Share on
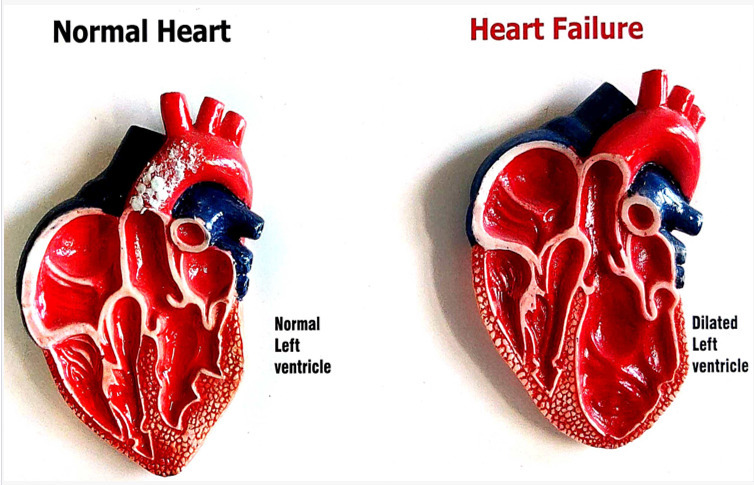
Advances in management of heart failure
www.bmj.com
April 24, 2024, 2:14 a.m.
The management of patients with heart failure has changed markedly in the past several years, with evidence for four life prolonging classes of drugs for patients with reduced LVEF and the benefit of SGLT2 inhibitors for those with mildly reduced and preserved LVEF. Device management and other non-drug management have evolved as results from new clinical trials are published. Identification of appropriate candidates for treatment requires accurate diagnosis, which can be challenging for patients with heart failure and preserved ejection fraction. Additional questions remain—in particular, how best to implement these new treatment recommendations into clinical practice.
Share on

Trends in the Management of Anterior Mitral Leaflet Regurgitation
jamanetwork.com
April 24, 2024, 2:10 a.m.
In this cross-sectional study, MV repair was the preferred option for degenerative mitral valve disease but was only slightly more commonly performed than MVR for isolated anterior leaflet pathologic status. A large proportion of MVR was performed without an MV repair attempt, suggesting reluctance to repair this pathologic condition.
Share on

Survey Finds Around 3 in 10 Adults Unaware of Common Lifestyle Factors that Impact Heart Health
www.prnewswire.com
April 24, 2024, 2:07 a.m.
Many people overlook common lifestyle factors that impact their heart health. The survey, conducted by Ipsos, reveals around three in 10 adults are unaware that alcohol (32 percent), smoking (27 percent), and lack of sleep (27 percent) are linked to heart disease.
Share on

Trends in the Management of Anterior Mitral Leaflet Regurgitation
jamanetwork.com
April 16, 2024, 9:17 a.m.
In this cross-sectional study, the surgical treatment of anterior leaflet abnormalities most commonly was noted with concomitant surgical procedures, and MV repair was only slightly more commonly performed than MVR. No attempt at MV repair was made before MVR in 81.7% of MVR cases. Neochordae repair and annuloplasty emerged as the predominant approach. Repair rates were higher in centers with higher volumes of anterior leaflet cases. There appear to be opportunities for improvement in the management of anterior leaflet abnormalities by the cardiac surgical community.
Share on

First large bore venous patient treated with PerQseal Elite vascular closure system
cardiovascularnews.com
April 16, 2024, 9:15 a.m.
Leveraging Vivasure Medical’s PerQseal technology, the PerQseal Elite vascular closure system is designed exclusively for sutureless and fully absorbable large-bore venous vessel closure following percutaneous cardiovascular procedures, such as transcatheter mitral valve repair or replacement (TMVR), transcatheter tricuspid valve repair or replacement (TTVR) and leadless pacemaker implants.
Share on

Long-Term Mortality after Transcatheter Edge-to-Edge Mitral Valve Repair Significantly Decreased over the Last Decade
www.mdpi.com
April 15, 2024, 1:31 p.m.
Different predictors for long-term mortality have been identified: while severe tricuspid regurgitation and NYHA class IV at baseline remained independently associated with an increased long-term mortality over the past decade, severe left ventricular dilatation and severe pulmonary hypertension predicted long-term mortality only in patients treated in the early M-TEER era. Although current guidelines recommend the procedure only for patients who remain symptomatic despite optimal medical therapy, our results suggest that timely treated patients benefit most from M-TEER before the onset of irreversible cardiac remodeling and severe pulmonary hypertension.
Share on

Complications in Acute Myocardial Infarction: Navigating Challenges in Diagnosis and Management
www.mdpi.com
March 24, 2024, 7:35 p.m.
The complications of AMI represent high-acuity, time-sensitive conditions associated with elevated morbidity and mortality rates. Early recognition and decisive intervention remain the cornerstones in the quest for optimal patient outcomes. Given the dramatic presentations associated with complications and the urgent need for intervention, early revascularization has become the standard of care, resulting in a reduced incidence of complications of less than 0.1%. Furthermore, emphasizing patient-centered planning and the judicious timing of appropriate interventions, including surgical procedures, percutaneous technologies, and mechanical circulatory support involvement, holds the potential to significantly enhance both disease- and patient-centered outcomes (Table 1). As a result, a multidisciplinary heart team emerges as a crucial entity in guiding the care of patients post-AMI with complications.
Share on

SS Innovations’ SSi Mantra Surgical Robotic System used to perform Mitral Valve Replacement
www.dicardiology.com
March 24, 2024, 7:33 p.m.
The SSi Mantra Surgical Robotic System, the first surgical robotic system to be made in India, and one of the few cost-effective global options with a wide range of surgical applications, has received regulatory approval in India, Indonesia and Guatemala, and is clinically validated for over 50 different types of surgical procedures. To date, more than 800 surgical procedures have been conducted using the system. SS Innovations has initiated the regulatory approval process in the United States and the EU, with approvals anticipated in the latter half of 2024 or 2025.
Share on

Impact of Frailty in Percutaneous Mitral & Tricuspid Valve Repair
www.physiciansweekly.com
March 24, 2024, 7:30 p.m.
Bleeding complications and infections were more frequent in frail patients undergoing transcatheter mitral and tricuspid valve repair and partly explained the longer hospital stay. Albeit some of the complications were associated with higher long-term mortality, this did not explain the strong association between frailty and mortality. Further research is warranted to explore interventions targeting periprocedural complications to improve outcomes in this vulnerable population.
Share on

What are the benefits of valve replacement surgery?
medserg.com
March 24, 2024, 7:28 p.m.
The heart, is a vital organ beating rhythmically, fuels life throughout our bodies. However, for millions worldwide, heart valve diseases disrupt this vital rhythm. Fortunately, recent years have witnessed groundbreaking progress in heart valve replacement surgery in India, offering renewed hope and an enhanced quality of life for those in need. Raising awareness about these advancements is crucial for optimal utilization and patient benefit.
Share on

Benefits of On-X Mitral Valve Replacement in Cases of Infective Endocarditis
www.hindawi.com
March 17, 2024, 3 p.m.
MVR performed with the On-X valve is an attractive surgical option for patients with mitral valve IE, with its associated mortality and morbidity rates being comparable to those associated with the use of the SJM valve plus its superior hemodynamic performance. EOA appears to be of independent predictive value for the occurrence of late-new-onset AF in patients who have undergone MVR for IE. The EOA achieved with the use of the On-X valve in the mitral position may reduce the incidence of new-onset AF in the late phase.
Share on

Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model
www.mdpi.com
March 17, 2024, 2:58 p.m.
We have successfully developed, tested, and, for the first time, implanted a novel H-TEHV featuring a permanent elastomeric scaffold in sheep. Our initial findings suggest that the H-TEHV demonstrates resilience under high-pressure conditions at the mitral position, exhibiting no signs of shrinkage or valve decomposition. While these short-term results may suggest the feasibility of the H-TEHV concept in vivo, it is crucial to acknowledge that given the current stage of development and the limited scope of a single animal implant, no definitive conclusions can be drawn regarding the long-term suitability of the H-TEHV.
Share on

Lithotripsy for Mitral Valve Stenosis: What's the Promise?
www.medscape.com
March 17, 2024, 2:55 p.m.
A novel technology called intravascular lithotripsy-facilitated percutaneous balloon mitral valvuloplasty (IVL-PBMV) shows promise for treating patients with severe calcific mitral stenosis (MS) and no other surgical or transcatheter treatment options. But until recently, evidence of its value has been sparse, consisting mainly of isolated case reports from various centers.
Share on

Complications in Acute Myocardial Infarction: Navigating Challenges in Diagnosis and Management
www.mdpi.com
March 17, 2024, 2:54 p.m.
The complications of AMI represent high-acuity, time-sensitive conditions associated with elevated morbidity and mortality rates. Early recognition and decisive intervention remain the cornerstones in the quest for optimal patient outcomes. Given the dramatic presentations associated with complications and the urgent need for intervention, early revascularization has become the standard of care, resulting in a reduced incidence of complications of less than 0.1%. Furthermore, emphasizing patient-centered planning and the judicious timing of appropriate interventions, including surgical procedures, percutaneous technologies, and mechanical circulatory support involvement, holds the potential to significantly enhance both disease- and patient-centered outcomes (Table 1). As a result, a multidisciplinary heart team emerges as a crucial entity in guiding the care of patients post-AMI with complications.
Share on